A Rational to Degradation of the P4 Tetrahedron
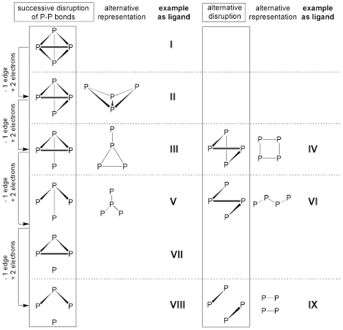
The most part of structures derived of transition metal complexes with „naked“ phosphorus ligands Pn (n≤4) can be rationalised by stepwise opening of edges in the P4 tetrahedron. For each of the above structural motives, ligands incorporated in transition metal complexes are described.
The formal opening of an edge can also be regarded as step-wise reduction of P4 with electron pairs forming (poly-)phosphide anions. Some of these are characterised as Zintl ions.
Study Targets
Understanding Transition Metal-Main Group element interaction. To this aim, the complexes from the interaction of (unsaturated) transition metal complexes are synthesised and characterised.
This concept is currently extended to the interaction of unsaturated main group compounds with elemental phosphorus.
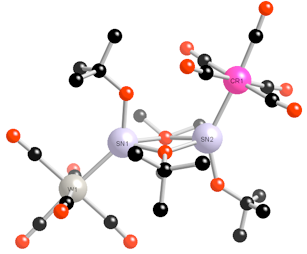
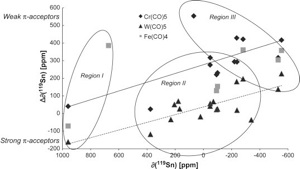
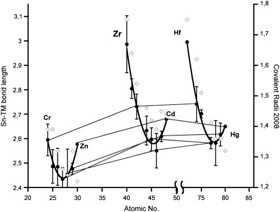
Molecular Structures